Introduction
We continue the SUES Forum with an attempt to broaden the scope of our material and start with a scientific item from Alan Potter on the chemical elements. This triggered off a memory of Tom Lehrer’s patter song on the same theme, so we have included some reference to the song and its author. Finally, Roger continues with his contributions on buildings, this time with an emphasis on the American Gilded Age. There are plentiful illustrations, but if you want to see even more examples, particularly from California, Roger has prepared a short Powerpoint that is being emailed along with this edition. Unfortunately we are unable to send Powerpoint material by post.
We are grateful for the messages of support we have received with regard to this venture and as this is a forum we feel it appropriate to share some of your responses.
Simply Elementary
Alan Potter
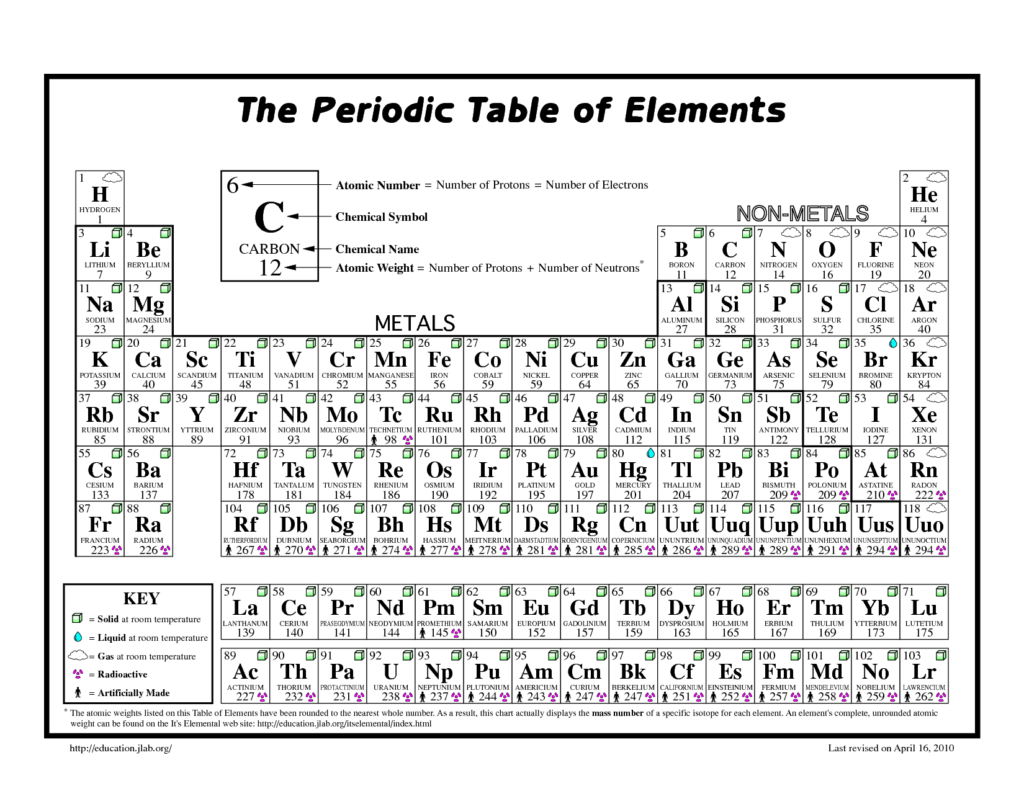
We have all probably used the term ‘element’ in everyday conversation but for scientists in general, and chemists in particular, a chemical element is a species of atom which has the same number of proton particles at its centre (nucleus). For example, the atomic number of nitrogen is seven, so the element nitrogen is made up of atoms, all of which have seven protons at their centre. The discovery of different elements has taken place over centuries but has accelerated over the last 100 years due to the design and manufacture of ever more sophisticated instruments and machinery.
To date, a total of 118 elements have been identified on Earth. The first 94 occur naturally and the remaining 24 are synthetic elements that have been made in laboratories. There are 80 elements that have at least one stable form, or isotope, while 38 that are exclusively radioactive and therefore decay away over time into other elements. The most abundant element (by mass) making up the Earth is iron, while oxygen is the most common element in the Earth’s crust. The two lightest elements, hydrogen and helium, were mostly formed at the creation of the universe, in the ‘Big Bang’, and are the most common elements that we know of in the universe. Although these chemical elements constitute all of the ordinary matter on Earth, they are only some of that in the universe as a whole. Astronomers contend that our observable substances make up only about 15% of the actual matter in the universe. The remainder is termed ‘dark matter’ – the composition of which is still unknown today.
The properties of all the chemical elements are summarised in the periodic table, which organises the elements by increasing atomic number into rows (called periods) in which the columns (called groups) share recurring physical and chemical properties. Apart from the unstable radioactive elements, which decay away quickly (have short half-lives), all of the elements are available to industry and most of them can be obtained with high purity. Considered by many as a design classic, the periodic table has been studied by children at schools for well over 100 years. However, to many, it has all the elegance of a well-designed material or wallpaper. Nevertheless, the way the table ranks atoms by their proton tally and then classifies them by electron arrangements is, first of all, a way of tidying up all the building blocks of matter. Alternatively, it can be seen as an incomplete jigsaw, where missing pieces indicate gaps for entirely new elements. The challenge used to be one of discovery where elements such as germanium were tracked down to slot in. But go past uranium (number 92) and outsize atoms get too unstable to exist naturally and have to be invented.
In recent years, a Japanese team got the credit for creating element 113, while a Russian/American team in California scored an atomic hat-trick, filling three missing numbers: 115, 117 and 118. Synthesized elements can have their uses and americium, for example, saves lives every day in smoke alarms. However, for most invented elements, the new atoms exist too fleetingly to be of immediate practical use. Nevertheless, in creating them, scientists do reaffirm the sturdiness of the table, and learn a good deal along the way. When the International Union of Pure and Applied Chemistry (IUPAC) announced the addition of these four new elements to the periodic table in 2016, the chart’s seventh row was officially complete. That marked the first time that new elements had been added to the table since 2011, when it was updated to include elements 114 (Flerovium) and 116 (Livermorium).
When the last four new elements were recognised, the scientists responsible for finding them could officially name them. Previously, the four elements were referred to by their tongue-twisting placeholder names: ununtrium (element 113), ununpentium (element 115), ununseptium (element 117), and ununoctium (element 118). According to the IUPAC, any new element can be named after their own properties (such as chlorine or barium), mythological concepts (such as thorium or vanadium), minerals (such as calcium or silicon), places (such as yttrium or californium) or countries (such as polonium or copper), or scientists (such as einsteinium or curium). There are still more new elements to be discovered and it is exciting to live in a time when mankind has the ability and the ingenuity to do so. However, at the same time, it is sobering to think that such good and potentially beneficial scientific work often goes unreported to, and unheralded by, the public at large.
The Elements Song
Alan Potter
The Elements is a song was written, in 1959, by musical humorist Tom Lehrer. He was also a mathematics lecturer at Harvard University. In the song he recites the names of all the chemical elements known at the time, 102, although they are not in the order outlined in the Periodic Table. The song is to the tune of The Major-General’s Song by Gilbert and Sullivan from their comic opera, The Pirates of Penzance. Here it is below:
There’s antimony, arsenic, aluminum, selenium
And hydrogen and oxygen and nitrogen and rhenium
And nickel, neodymium, neptunium, germanium
And iron, americium, ruthenium, uranium
Europium, zirconium, lutetium, vanadium
And lanthanum and osmium and astatine and radium
And gold and protactinium and indium and gallium
And iodine and thorium and thulium and thallium
There’s yttrium, ytterbium, actinium, rubidium
And boron, gadolinium, niobium, iridium
And strontium and silicon and silver and samarium
And bismuth, bromine, lithium, beryllium and barium
Isn’t that interesting? I knew you would. I hope you’re all
taking notes because there’s going to be a short quiz next period
There’s holmium and helium and hafnium and erbium
And phosphorous and francium and fluorine and terbium
And manganese and mercury, molybdenum, magnesium
Dysprosium and scandium and cerium and caesium
And lead, praseodymium and platinum, plutonium
Palladium, promethium, potassium, polonium
And tantalum, technetium, titanium, tellurium
And cadmium and calcium and chromium and curium
There’s sulphur, californium and fermium, berkelium
And also mendelevium, einsteinium, nobelium
And argon, krypton, neon, radon, xenon, zinc and rhodium
And chlorine, carbon, cobalt, copper, tungsten, tin and sodium
There are the only ones of which the news has come to Harvard
And there may be many others but they haven’t been discovered
We know that all elements are named according to the International Union of Pure and Applied Chemistry (IUPAC) and that any new element can be named after:
1. their own properties
2. mythological concepts
3. minerals
4. places
5. countries
It may be interesting to find out which of the five naming methods were used for each element. For example, four were named after a small town in Sweden. Good luck!
Tom Lehrer
John Sharp

Radio 3 recently celebrated Tom Lehrer’s 92nd birthday by playing some of his most popular numbers, including ‘Poisoning Pigeons in the Park’, the title of which will make it clear that Lehrer has a strong strain of black humour. His is a remarkable career. He started playing the piano at 7 and then entered Harvard to study mathematics at the age of 15, being regarded as a prodigy. Lehrer saw his interests in mathematics and music as complementary, both involving precision and logic. He taught mathematics at the Massachusetts Institute of Technology and the University of California and at one time worked for NASA. At the same time he wrote songs and appeared in satirical TV programmes, including the US equivalent of ‘That was the week that was’. His songs were recorded and achieved a world-wide popularity in the 1950s and 1960s. They are a mixture of parody, mockery, social commentary, black humour, brilliant word-play and bravura performance. When the New York Times commented ‘Mr Lehrer’s muse is not fettered by such inhibiting features as taste’, he was delighted!
If you have never heard his songs – and are not put off by bad taste – they are worth trying. You will find some on YouTube. The Elements Song, which isn’t in bad taste, is a showpiece of memory and technique. Just try to sing along with it and you’ll see what I mean. Interestingly, this was the only number in which he adapted someone else’s melody.
American Houses of the Gilded Age: 1875 – 1910 – The Queen Anne Style
Roger Mitchell
This is the period in which some of America’s biggest and grandest houses were built including most of the Newport ‘Cottages’, as well as the grand houses on Long Island and up the Hudson Valley north of New York. We will explore some of these in a later Forum but in this first look at the architecture of the Gilded Age, I want to concentrate on more modest buildings. We could describe them as true cottages, although they are probably a bit too large and were essentially middle-class homes. We can find them right across the continent. Some are built of brick, but the most common building material is wood and carpenters had a field day, decorating and ornamenting the houses. They are informal, asymmetrical, eclectic and really ought to be called Victorian (a widely accepted art historical term in America). The picture below comes from one of my favourite books, ‘A Cartoon History of Architecture’ by Osbert Lancaster. The title for this picture is ‘American Queen Anne’ which Lancaster himself describes as ‘hard to justify’.
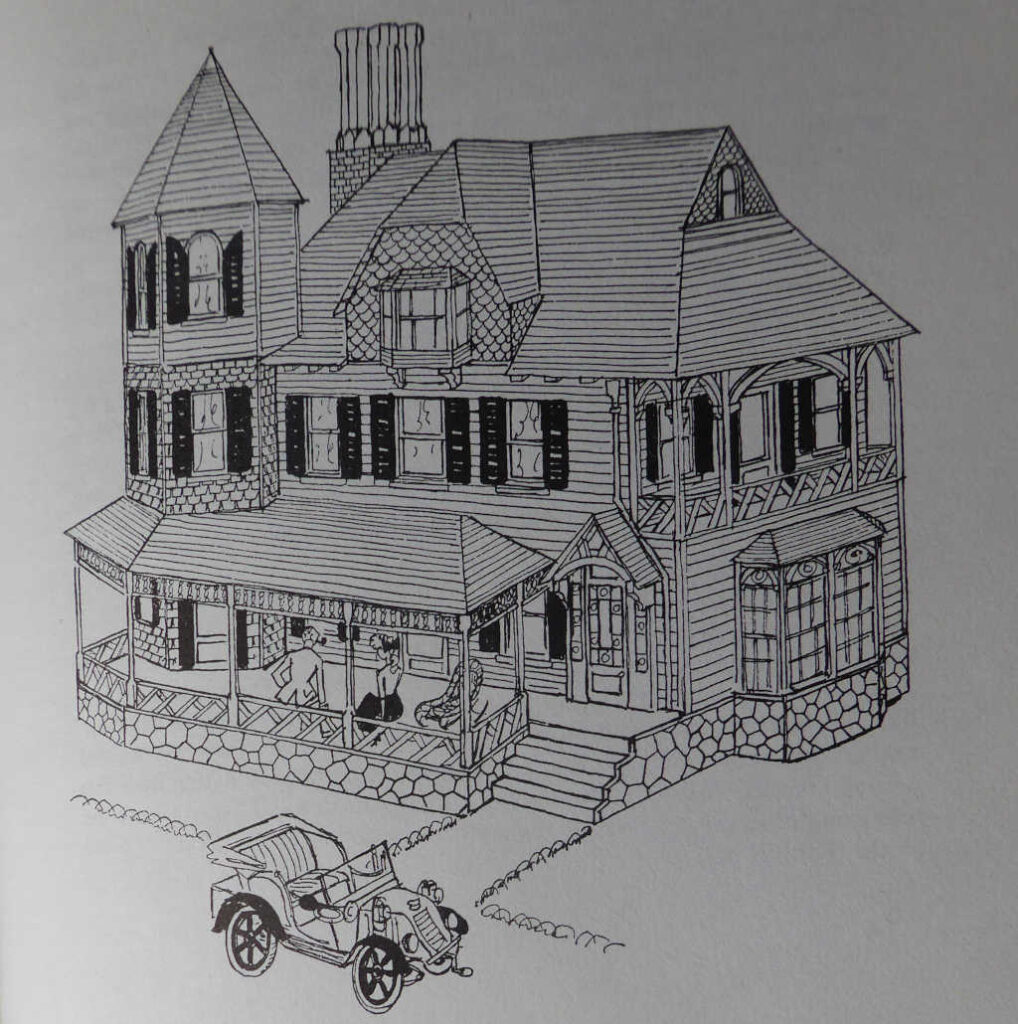
However, this is the usual term that Americans use to describe these houses, even though ‘Queen Anne’ seems to make absolutely no sense at all. ‘Real’ Queen Anne (i.e. English houses of the early 18th century) look like this.

Or like this:

Or like this. These are formal, symmetrical manor houses and rectories mostly built of brick. There is a hint of the Baroque but very much toned down compared with Blenheim Palace or Castle Howard.

American Queen Anne houses look very different. The house below is built of brick and therefore has something in common with the English houses. Even so, it is completely asymmetrical and the ground floor design is dominated by porches and verandas – a very American feature.

All the other American houses that follow use timber as the main building material. Perhaps timber encourages elaborate decoration, full of towers and gables and willing to try anything – just look at the entrance porch for this house in California.

The two houses below are rather less extreme. The top picture shows a house in North Carolina where long, humid summers encourage the use of porches and verandas with as much protection from the sun as possible. The bottom picture shows a house in Vancouver, Washington State. It is an army house built for the commanding officer and his family and it would look right and appropriate anywhere in America.


The house shown below does not even try to fit in. It wants to dominate its surroundings and show the success and the wealth of its builder. It is the Carson Mansion in Eureka, California built in 1884 for William Carson who had made his money from logging. Its website is entirely happy to describe its architectural style as Queen Anne.


Carson Mansion really is a monster, but ‘once seen, never forgotten’. You can do a guided tour online at ingomar.org and then for something a little more restful go to Thomasville, Georgia to explore the Lapham/Patterson House which was also built in the mid-1880s as a winter residence for a Chicago shoe shop owner. There is a picture below, but if you go to the Georgia State Parks website, you can watch a 14 minute video of the house. Your guide is the House Curator who showed us round when we went there ten years ago.

You should now have a reasonable idea of what ‘Queen Anne Style’ looks like, but you will probably still be puzzled as to how they acquired that confusing name. The English architect Richard Norman Shaw is partly to blame. In the early 1870s, he and some of his contemporaries developed a style for town houses that was derived chiefly from 17th century English and Dutch precedents. It was a lively and original style with a fondness for red brick, white woodwork, prominent chimneys, high roofs and Flemish gables. Mark Girouard has written a whole book about it – Sweetness and Light: The Queen Anne Movement 1860-1900 and he describes it as ‘a kind of architectural cocktail with a little genuine Queen Anne in it, a little Dutch, a little Flemish, a squeeze of Robert Adam, a generous dash of Wren and a touch of Francis I’. The Queen Anne label was applied by contemporaries and the style is best seen in West London (Kensington and Chelsea) and at the seaside – some of Southport’s villas would qualify. American architects took elements back across the Atlantic. They kept the name ‘Queen Anne’ but, as we have seen they were quite happy to make much greater use of timber, which encouraged elaborate decoration, and to add new elements, porches and verandas in particular. Perhaps the best guidance is not to worry about the name, but just enjoy the fun and the exuberance.
However, you may feel that these idiosyncratic houses are not serious pieces of architecture and so as a final house, I have chosen something that I think is more serious and extremely successful. It is the Isaac Bell House in Newport, Rhode Island designed by Stanford White and now owned by The Preservation Society of Newport County (see their website for more information). Even though the house is described as being part of the Queen Anne revival, it is specifically praised as ‘an American Shingle Style Masterpiece’ because the architectural firm of McKim, Mead & White were inspired to look to colonial American buildings for inspiration. Many of the colonial buildings of New England had a direct impact on their quest to design a truly “American” style of house.


Responses
Andrew Wilcockson draws our attention to a book he stumbled across recently (on Abe Books): Religion and Place: Liverpool’s historic places of worship (Informed Conservation) by Brown, Sarah; Figuereido, Peter De: Very Good Soft cover (2008) Stephen Books
Ron and Sue James tell us, ‘being ‘hibernating’ gives us time to read the papers all the way through and make a start on preparing the greenhouse and the plants to brighten up the garden in the summer. The garden gives us the opportunity to ‘take the air’ at home during these difficult days.’
Judy Hermida doesn’t ‘mind at all living like “a hermit” in these very strange and worrying times. I am working from home for The City of Liverpool College, and am quite content to work in solitude – for the time being at least, that is.’
On the other hand, Roger Allison reflects that ‘on top of a high pillar I might escape the endless propaganda about Covid-19, but would not be able to enjoy the SUES programme of events… Pray that house arrest ends soon.’
Contacts
Chair: Alan Potter
alanspotter@hotmail.com
07713 428670
Secretary: Roger Mitchell
rg.mitchell@btinternet.com
01695 423594 (Texts preferred to calls)
Membership Secretary: Rob Firth
suesmembers74@gmail.com
01704 535914
Forum Editor: Chris Nelson
chris@niddart.co.uk
07960 117719
Facebook: facebook.com/groups/southportues
See our archive for previous editions of the SUES Forum!